Product
Mycobacteria Identification Kit (Sanity 2.0)
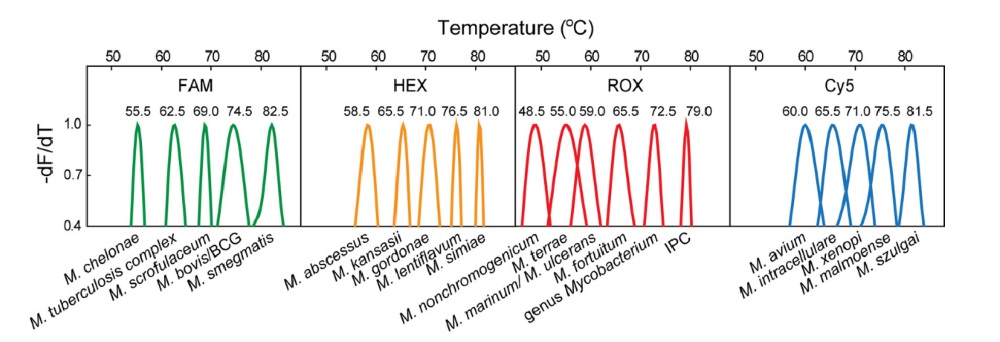
The Mycobacterium kit is capable of identifying 19 clinically relevant mycobacteria in a single reaction, including common non-tuberculous mycobacteria (NTM) and the Mycobacterium tuberculosis complex (MTBC). It is able to accurately identify 51 reference mycobacterial strains to the species/genus level and exhibits no cross-reactivity within NTM and MTBC strains[1].
Fig.1 Melting peaks for each species in the Mycobacterium kitin line with the 2D label strategy[1].
The kit is intended for use with the Sanity 2.0 System, which is a fully automated system that simplifies the testing process. It combines nucleic acid extraction, real-time polymerase chain reaction (rt-PCR), and result interpretation into a single, streamlined operation.
Principle
Mycobacteria Identification Kit (Sanity 2.0) employs Multicolor Melting Curve Analysis (MMCA), a technique that uses dual-labeled probes to detect excess single-stranded DNA amplicons generated through asymmetric PCR. This method integrates multiple fluorescence colors with melting curve analysis to accurately detect and differentiate various target sequences. The specific melting temperature (Tm) obtained from this analysis provides sequence information. When a target of interest is present, a melting peak appears at a characteristic position, and the Tm value correlates with specific mycobacterial species.
Additionally, the test includes a fragment of the Arabidopsis thaliana sucrose-proton symporter 2 (SUC2) gene, which serves as an internal Positive Control (IPC). This control indicates whether PCR inhibition has occurred, ensuring the reliability of the test results.
Features
- Single-tube multiplex real-time PCR powered by patented MMCA/MeltArray technology
Simultaneous detection of 52 species Mycobacteriam in a single-tube reaction.
A total of 52 species will be categorized into 19 distinct groups.
- Ease of use
Automated result interpretation through Sanity 2.0 System.
Pre-loaded lyophilized reagents for easy handling and room-temperature transportation.
- Fast and efficient
Turnaround time is less than 3 hours for 51 targets.
Sample Type
Sputum
Mycobacterium tuberculosis culture
Compatible Kit
This kit should be used with MTB DNA Extraction Kit (Sanity 2.0) for nucleic acid extraction.
CE-IVD Marked
[1]Xu Y, Liang B, Du C, Tian X, Cai X, Hou Y, Li H, Zheng R, Li J, Liu Y, Wang K, Ammar Athar M, Tan Y, Li Q. Rapid Identification of Clinically Relevant Mycobacterium Species by Multicolor Melting Curve Analysis. J Clin Microbiol. 2019 Jan 2;57(1):e01096-18. doi: 10.1128/JCM.01096-18.
Interested to Know More about Our Solution to Molecular Diagnostics?