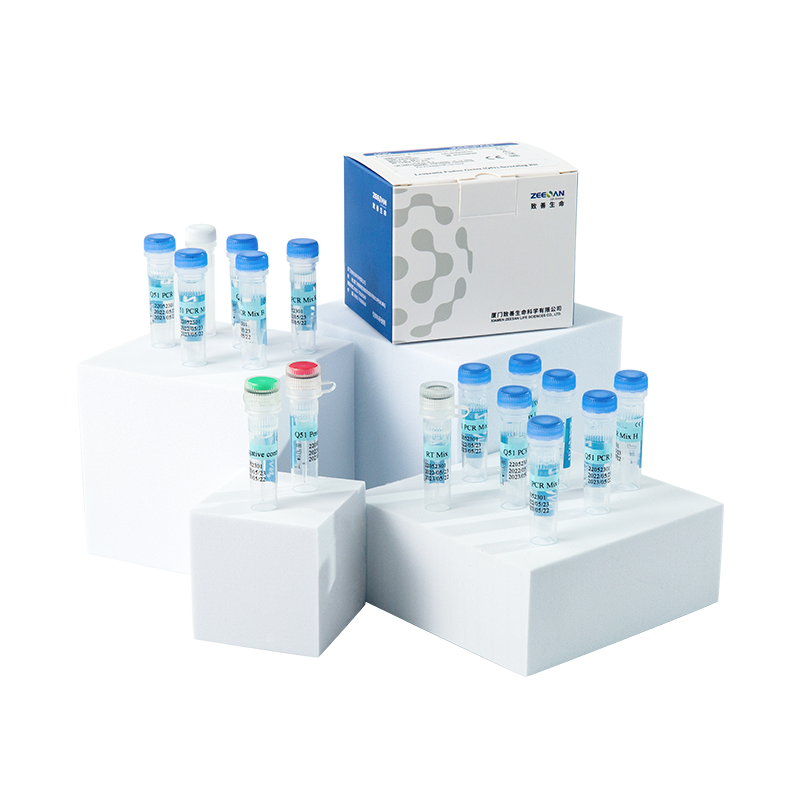
Product
Leukemia Fusion Genes (Q51) Screening Kit
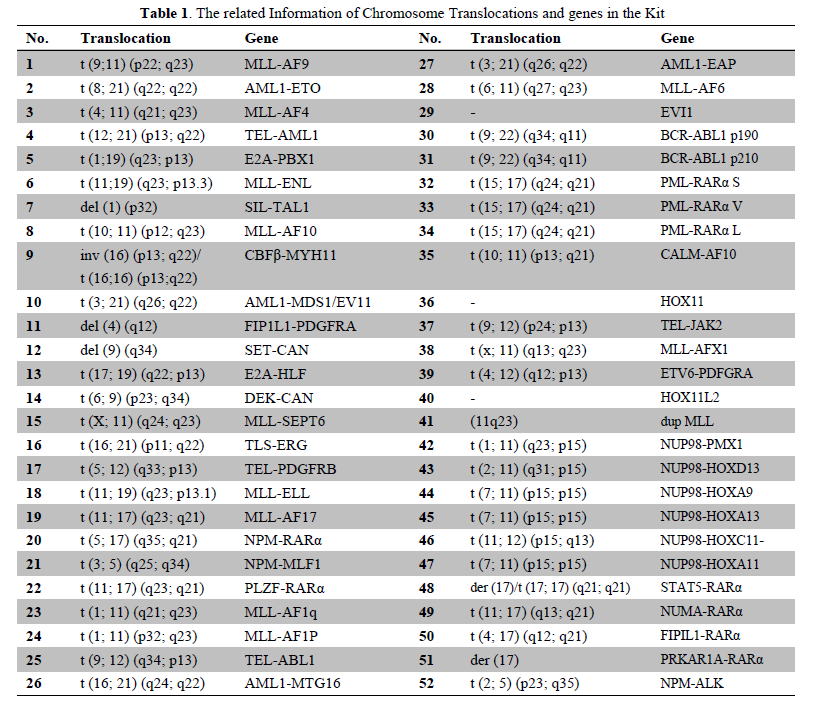
Leukemia Fusion Genes (Q51) Screening Kit is a qualitative in vitro diagnostic test for screening of 49 fusion genes resulted from chromosome translocations involved in chronic and acute leukemia, and leukemia-associated genes EVI1, HOX11, HOX11L2(Table 1). All the 49 fusion genes include more than 182 clinically relevant chromosomal breakpoints. The kit detects RNA transcripts of the 52 leukemia-associated genes extracted from human bone marrow or whole blood samples using a RT-qPCR procedure. The results of the kit allow professionals to be more aware of the patient’s prognosis and provides professionals important insights into the treatment planning.
Principle
Leukemia Fusion Genes (Q51) Screening Kit is a multiplex RT-qPCR based assay for detection of the 52 leukemia-associated genes transcripts including EVI1, HOX11, HOX11L2 and 49 fusion genes in total RNA from bone marrow or whole blood samples. Included in the kit are RT reaction mix and qPCR mix. cDNA is synthesized by adding purified total RNA to the RT reaction mix prepared in advance. The resulting cDNA is added to 12 qPCR reaction tubes, which contain specific PCR primers and probes for detection of the 52 leukemia-associated genes and an internal control gene of GUSB. The qPCR is performed in a real-time thermal cycler with optical filters for the detection of FAM, ROX, HEX and Cy5 fluorescence signals. Amplification plots and the resulting Cq (quantification cycle) values are used for the identification of the 52 leukemia-associated genes transcript.
CE-IVD Marked
Interested to Know More about Our Solution to Molecular Diagnostics?